The Cellular Engine: Supercharging Mitochondria and Activating Autophagy
At the core of human health, performance, and aging lie two fundamental biological processes: the generation of energy and the maintenance of cellular integrity. The first pillar, cellular power, is governed by the mitochondria. The second, cellular purity, is orchestrated by a process known as autophagy. Understanding the profound synergy between these two systems is the key to unlocking unprecedented levels of physical and cognitive vitality. They are not separate functions but two sides of the same coin—a symbiotic dance of power and purity that dictates our capacity to thrive.
The Twin Pillars of Vitality: Power and Purity
Mitochondria are the sophisticated organelles responsible for aerobic respiration, the process that generates the vast majority of the cell's energy currency: adenosine triphosphate (ATP). Every muscle contraction and nerve impulse is fueled by ATP produced by these microscopic engines. However, a natural byproduct of this energy production is the creation of reactive oxygen species (ROS), or free radicals. When mitochondria become dysfunctional, they produce excessive ROS, leading to a state of oxidative stress that damages the cell and is a primary driver of the aging process.
This is where autophagy, the body's ultimate quality control system, becomes critical. Derived from the Greek for "self-eating," autophagy is the cell's intrinsic mechanism for identifying and eliminating damaged organelles and cellular debris.
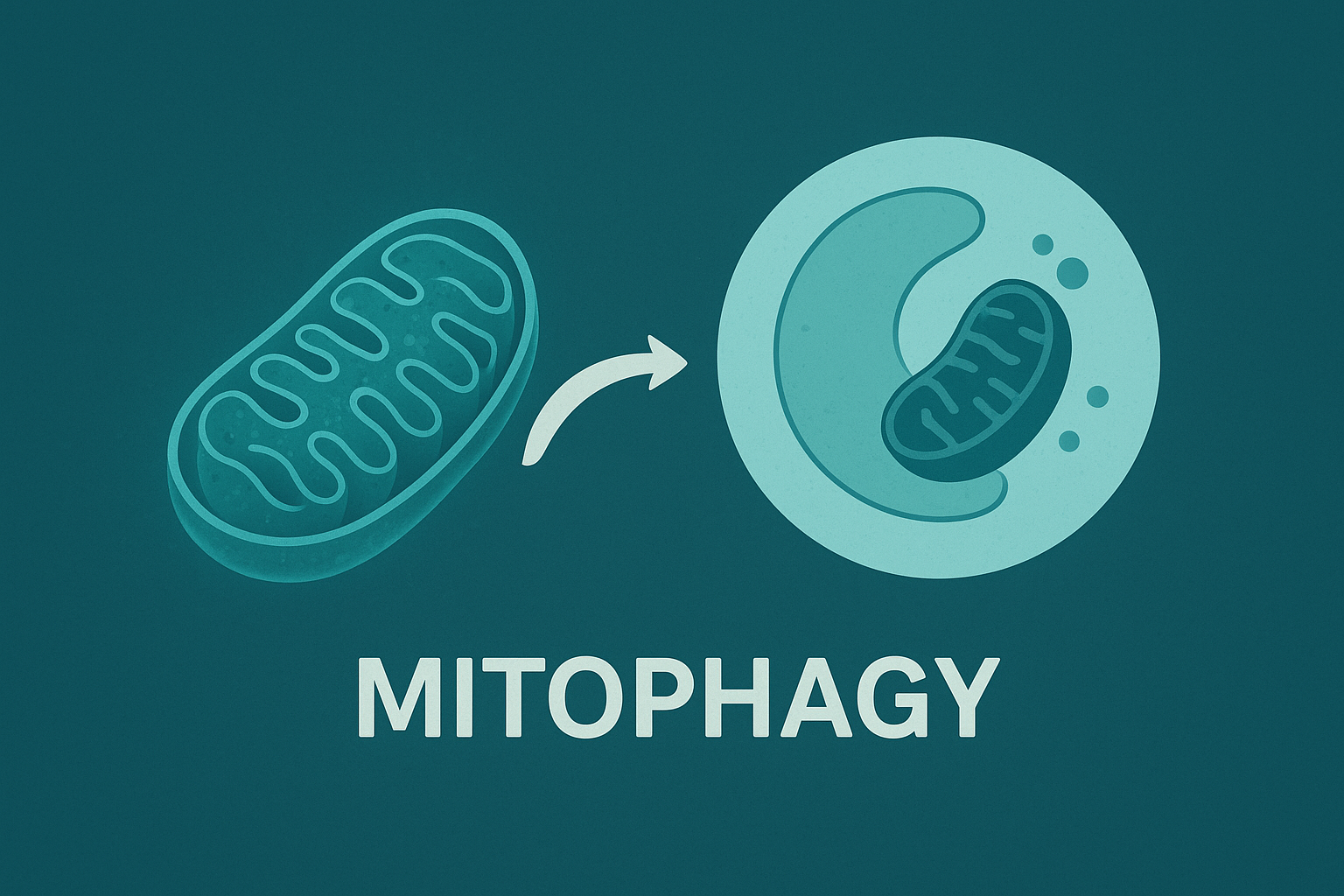
A specialized form of this process, mitophagy, specifically targets and removes damaged mitochondria. By clearing out the dysfunctional, ROS-producing mitochondria, mitophagy reduces oxidative stress and makes resources available for the creation of new, pristine ones. This dynamic balance is the key to maintaining a healthy, high-functioning cellular network. Remarkably, numerous scientific pathways known to extend lifespan in model organisms all converge on a single, required mechanism: the upregulation of autophagy.
Forging the Engine: How Exercise Builds a Superior Mitochondrial Network
Physical exercise is the single most potent, non-pharmacological tool for taking conscious control of this system. Exercise is a powerful stressor that initiates a cascade of profound adaptations, chief among them a complete remodeling of the mitochondrial network.
Exercise is the most powerful physiological stimulus for mitochondrial biogenesis—the process of creating new mitochondria. The cellular stress from a workout activates a master regulatory protein known as PGC-1α, which orchestrates the construction of new mitochondria. This adaptation isn't limited to muscle; regular exercise also increases markers of mitochondrial biogenesis in the brain and fat tissue, promoting whole-body metabolic health.
Different training styles yield complementary benefits. Endurance training is the gold standard for increasing mitochondrial quantity, while resistance training appears to enhance mitochondrial quality and efficiency. A combination of both forges a system that is both dense and powerful. Crucially, exercise is a powerful antidote to age-related mitochondrial decline, effectively reversing many deficits and coaxing older mitochondria to behave more like young, vibrant ones.
The Art of Cellular Renewal: Activating Autophagy
The same cellular stress from a workout that builds new mitochondria also kickstarts the profound renewal process of autophagy. The very same signaling molecules that are activated by exercise to promote mitochondrial biogenesis—such as AMPK and sirtuins—are also primary activators of the autophagy pathway. This means the cellular signal to "build new" is intrinsically linked to the signal to "clear out the old."
This cleanup process is essential for muscle to remodel and become stronger. Without a functioning autophagy system, the cellular debris generated during a workout accumulates, leading to muscle fiber degeneration and a failure to adapt to training. This reveals that exercise-induced autophagy is a crucial myoprotective (muscle-protecting) process required for safe and effective training adaptation. This entire process is a perfect illustration of hormesis, where a beneficial adaptation results from exposure to a mild, manageable stress.
Hacking the System: Fasting, Feasting, and Metabolic Flexibility
The cell's metabolic state is largely controlled by two key nutrient-sensing pathways: AMPK and the mechanistic target of rapamycin (mTOR).
mTOR is the primary growth-promoter, activated by an abundance of nutrients. When mTOR is active, it promotes cell growth and powerfully inhibits autophagy.
AMPK is the energy sensor, activated by low cellular energy during exercise or fasting. When AMPK is active, it activates autophagy to recycle components for fuel.
Practices like intermittent fasting (IF) and time-restricted eating (TRE) are potent methods for deliberately inhibiting mTOR and activating AMPK, thereby inducing a powerful autophagic response. Performing exercise in a fasted state can be a particularly potent stimulus, as it combines both the energy-depletion and nutrient-deprivation triggers that converge on the AMPK pathway.
The Biohacker's Toolkit: Product Recommendations
While exercise and fasting are foundational, specific supplements can further support and amplify these cellular processes. The following compounds can serve as powerful tools within a comprehensive protocol.
Disclaimer: This information is for educational purposes only and is not medical advice. Always consult with a qualified healthcare professional before beginning any new supplement regimen.
| Supplement | Primary Bio-Function | Key Insight |
|---|---|---|
| Coenzyme Q10 (as Ubiquinol) | ATP Production & Mitochondrial Antioxidant | Crucial for energy efficiency. Exercise can naturally increase muscle CoQ10 levels, which may be depleted by statin use. |
| PQQ (Pyrroloquinoline Quinone) | Mitochondrial Biogenesis | Acts synergistically with CoQ10; PQQ helps build more “engines” while CoQ10 helps them run better. Reduces key inflammatory markers. |
| Spermidine | Potent Autophagy Induction | Levels decline with age but are high in healthy centenarians. Essential for the full benefits of fasting-induced autophagy. |
| Trans-Resveratrol | Sirtuin Activation & Autophagy | A powerful “fasting mimetic” that works through a different but complementary pathway to spermidine. |
| EGCG (from Green Tea Extract) | Antioxidant & Autophagy Modulation | A versatile compound that supports cellular health by both protecting against damage and stimulating the cleanup process. |
Works Cited
Conley, K. E., et al. (2007). "Mitochondrial dysfunction and age." Current Opinion in Clinical Nutrition and Metabolic Care. https://pubmed.ncbi.nlm.nih.gov/18089948/
Lira, V. A., et al. (2013). "Autophagy is required for exercise training-induced skeletal muscle adaptation..." The FASEB Journal. https://pmc.ncbi.nlm.nih.gov/articles/PMC4796178/
Pizzorno, J. (2014). "Mitochondria-Fundamental to Life and Health." Integrative Medicine (Encinitas). https://pmc.ncbi.nlm.nih.gov/articles/PMC4684129/
Memme, J. M., et al. (2021). "Exercise and mitochondrial health." The Journal of Physiology. https://pubmed.ncbi.nlm.nih.gov/31674658/
Egan, B., & Zierath, J. R. (2013). "Exercise metabolism and the molecular regulation of skeletal muscle adaptation." Cell Metabolism. https://pmc.ncbi.nlm.nih.gov/articles/PMC6120690/
Hansen, M., et al. (2018). "Autophagy as a promoter of longevity: insights from model organisms." Nature Reviews Molecular Cell Biology. https://pmc.ncbi.nlm.nih.gov/articles/PMC5792715/
Wong, S. Q., et al. (2019). "Autophagy in aging and longevity." Human Genetics. https://pmc.ncbi.nlm.nih.gov/articles/PMC6884674/
Irving, B. A., et al. (2015). "Combining aerobic and resistance training increases training-induced mitochondrial adaptation..." Journal of Applied Physiology. https://pmc.ncbi.nlm.nih.gov/articles/PMC4478283/
Madeo, F., et al. (2015). "Essential role for autophagy in life span extension." Journal of Clinical Investigation. https://pmc.ncbi.nlm.nih.gov/articles/PMC6287690/
Morselli, E., et al. (2011). "Spermidine and resveratrol induce autophagy by distinct pathways..." Journal of Cell Biology. https://pmc.ncbi.nlm.nih.gov/articles/PMC2815753/
Di Meo, S., & Venditti, P. (2020). "Evolution of the knowledge of the role of reactive oxygen species in exercise." Journal of Experimental Biology. https://pmc.ncbi.nlm.nih.gov/articles/PMC8533813/
Hawley, J. A., & Bishop, D. J. (2025). "The impact of exercise on mitochondrial dynamics." American Journal of Physiology-Endocrinology and Metabolism. https://pubmed.ncbi.nlm.nih.gov/39441237/
Hood, D. A. (2009). "Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle." Applied Physiology, Nutrition, and Metabolism. https://pmc.ncbi.nlm.nih.gov/articles/PMC3384482/
He, C., et al. (2012). "Exercise induces autophagy in peripheral tissues and in the brain." Autophagy. https://pmc.ncbi.nlm.nih.gov/articles/PMC5538402/
Kim, Y., & Kim, Y. S. (2023). "Physiological roles of intermittent fasting in the body." Journal of the Korean Medical Association. https://pmc.ncbi.nlm.nih.gov/articles/PMC9946909/
Morales, P. E., et al. (2017). "The impact of exercise on mitochondrial biogenesis in skeletal muscle: A systematic review and meta-analysis..." Journal of Physiology and Biochemistry. https://pubmed.ncbi.nlm.nih.gov/40459444/
Murton, A. J., & Carter, H. N. (2024). "Autophagy and skeletal muscle health in aging and disease." Journal of Cachexia, Sarcopenia and Muscle. https://pmc.ncbi.nlm.nih.gov/articles/PMC12401363/
Alirezaei, M., et al. (2010). "Short-term fasting induces profound neuronal autophagy." Autophagy. https://pmc.ncbi.nlm.nih.gov/articles/PMC3106288/
Hofer, S. J., & Madeo, F. (2022). "Mechanisms of spermidine-induced autophagy and geroprotection." Nature Aging. https://pubmed.ncbi.nlm.nih.gov/37118547/
Tarnopolsky, M. A. (2007). "Mitochondrial dysfunction: impact on exercise performance and cellular aging." Exercise and Sport Sciences Reviews. https://pubmed.ncbi.nlm.nih.gov/17417049/
Leduc-Gaudet, J. P., et al. (2021). "Mitochondrial Dynamics and Quality Control in Skeletal Muscle: An Overview." Frontiers in Physiology. https://pmc.ncbi.nlm.nih.gov/articles/PMC8110831/
Kim, Y. A., et al. (2018). "The role of autophagy in skeletal muscle." Journal of Exercise Nutrition & Biochemistry. https://pmc.ncbi.nlm.nih.gov/articles/PMC6386226/
Rubinsztein, D. C., et al. (2011). "Autophagy and aging." Cell. https://pmc.ncbi.nlm.nih.gov/articles/PMC2755611/
Pietrocola, F., & Madeo, F. (2023). "Spermidine is essential for fasting-mediated autophagy and longevity." Cell Reports. https://pubmed.ncbi.nlm.nih.gov/39117797/